COVID 'vaccines' are a medical experiment on humanity
- describes the risks, but even prior to the risk factors - consider these facts: The vaccines aren't designed to prevent COVID. An FDA Pfizer briefing paper published December 10, 2020, revealed 43 percent more suspected cases of Covid-19 in the vaccinated group than in the placebo group within seven days of vaccination. They will also not end restrictions. Dr. Anthony Fauci of the National Institutes of Health acknowledges that the vaccines may prevent symptoms but will not block spread of the virus, so vaccine recipients will still need to wear masks, practice social distancing and avoid crowds.
Then it's not a vaccine: Crazy Dr. Fauci said in October early COVID vaccines will only prevent symptoms and NOT block the Infection ...What?
HUNDREDS of Israelis get infected with Covid-19 after receiving Pfizer/BioNTech vaccine
Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions https://www.sciencemag.org/news/2020...gic-reactions?
Why Did a COVID Vaccine Turn HIV Tests Positive? https://articles.mercola.com/sites/a...rid=1049633063
"I'm Choosing The Risk Of Getting COVID": Over Half Of Health Care Workers At California Hospitals Refuse Vaccinations https://www.zerohedge.com/covid-19/i...spitals-refuseStory at-a-glance
In the race to produce a viable vaccine for COVID-19, one developed at the University of Queensland, Australia, was scrapped when scientists found participants developed a false positive test for HIV after vaccination
Researchers who had used recombinant adenovirus type 5 (Ad5) vector 10 years ago for an HIV-1 vaccine warned against using the same process for the development of a COVID-19 vaccine as it raised the risk of an HIV infection
It is notoriously difficult to develop a vaccine for coronaviruses as the process has always backfired in the past, raising the risk of severe disease when exposed to the virus
Current data show a mass vaccination mandate is not necessary; pharmaceutical companies are shielded from liability from vaccine injury. Weigh your personal risks and benefits before deciding
Twitter blocks European MEDICAL JOURNAL after it published study on promising ivermectin treatment for Covid-19
Not the best interview (or may be an editing issue) - confusing time lines, but really good people. Video @ 11 minutes looks at how we can counteract insanity - one group has process in place to identify organism in cases of death: Covid “Dark Winter”: Political Announcements of “Highly Contagious Virus Variants” https://www.globalresearch.ca/covid-...riants/5733458
-
 Bulletin Board
Quick Nav
Bulletin Board
Quick Nav

- Bulletin Board
- Sonoma County Bulletin Board
- Coronavirus Conspiracy Theories
- More about vaccines
So Long and Thanks for All the Fish!
This site is now closed permanently to new posts.We recommend you use the new Townsy Cafe!
Click anywhere but the link to dismiss overlay!
- Share this thread on:
- Follow: No Email
-
Thread Tools
-
Search Thread
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
More about vaccines
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
NOTE: PEG i.e. polyethylene glycol is the main ingredient in antifreeze.
Also... it is in many medications:
https://www.gutsense.org/gutsense/th...alzheimer.html
 December of 2011, the FDA placed MiraLAX — a polyethylene glycol-containing blockbuster drug marketed by Merck & Co — on its Adverse Event Reporting System (AERS) in connection to “neuropsychiatric events.” Besides MiraLAX, this warning also applies to Movicol, Dulcolax, Colyte, Colovage, Co-Lav, Clensz-Lyte, ClearLax, GoLYTELY, GaviLyte C, GlycoLax, Go-Evac, GlycoPrep, E-Z-Em Fortrans, Halflytely, Lax-a-Day, LaxLyte, MoviPrep, Macrogol, NuLytely, OCL, Peg-Lyte, Prep Lyte, Softlax, TriLyte, and all other brands with Polyethylene Glycol 3350 (PEG for short) as their active ingredient.
December of 2011, the FDA placed MiraLAX — a polyethylene glycol-containing blockbuster drug marketed by Merck & Co — on its Adverse Event Reporting System (AERS) in connection to “neuropsychiatric events.” Besides MiraLAX, this warning also applies to Movicol, Dulcolax, Colyte, Colovage, Co-Lav, Clensz-Lyte, ClearLax, GoLYTELY, GaviLyte C, GlycoLax, Go-Evac, GlycoPrep, E-Z-Em Fortrans, Halflytely, Lax-a-Day, LaxLyte, MoviPrep, Macrogol, NuLytely, OCL, Peg-Lyte, Prep Lyte, Softlax, TriLyte, and all other brands with Polyethylene Glycol 3350 (PEG for short) as their active ingredient.
According to the National Institute for Occupational Safety and Health, it is an extremely toxic substance: “Ethylene glycol is chemically broken down in the body into toxic compounds. It and its toxic byproducts first affect the central nervous system (CNS), then the heart, and finally the kidneys. Ingestion of sufficient amounts [as little as 30 ml — KM] can be fatal.” [2]
The term “neuropsychiatric events” in the FDA's safety alert refers to neurologic disorders of the central and peripheral nervous systems such as autism, dementia, depression, schizophrenia, multiple sclerosis, Alzheimer’s and Parkinson’s diseases, and similar others [3]. These conditions result from PEG's direct (through cellular damage) and indirect (through malnutrition of essential micronutrients) neurotoxicity. No surprise there considering the quotation above.
Lead, mercury, and arsenic are some of the best-known neurotoxins. So are snake venom, curare, botulinum, and tetanus. PEG is more like lead or mercury — slow-acting, insidious, and difficult to pin down conclusively onto various “slow-brewing” neurological disorders.
In addition to neurotoxicity, the following serious complications have been associated with polyethylene glycol-containing laxatives: see article for rest....
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
COVID 'vaccines' are a medical experiment on humanity
describes the risks, but even prior to the risk factors - consider these facts: The vaccines aren't designed to prevent COVID. An FDA Pfizer briefing paper published December 10, 2020, revealed 43 percent more suspected cases of Covid-19 in the vaccinated group than in the placebo group within seven days of vaccination. They will also not end restrictions. Dr. Anthony Fauci of the National Institutes of Health acknowledges that the vaccines may prevent symptoms but will not block spread of the virus, so vaccine recipients will still need to wear masks, practice social distancing and avoid crowds.
Then it's not a vaccine: Crazy Dr. Fauci said in October early COVID vaccines will only prevent symptoms and NOT block the Infection ...What?
"I'm Choosing The Risk Of Getting COVID": Over Half Of Health Care Workers At California Hospitals Refuse Vaccinations https://www.zerohedge.com/covid-19/i...spitals-refuse
Canadian personal support worker suffers 'rare but severe' reaction to Pfizer COVID-19 vaccine
Health authorities on alert after nurse DIES following vaccination with Pfizer’s Covid-19 shot in Portugal https://www.rt.com/news/511524-portu...fizer-vaccine/
2 people die in Norway nursing home days after Pfizer's Covid-19 vaccine, investigation launched
A 'healthy' doctor died two weeks after getting a COVID-19 vaccine https://www.chicagotribune.com/coron...2py-story.html
same doctor - different article: 'Perfectly healthy' doctor dies of a blood disorder 16 days after Covid-19 vaccine; wife certain it was jab triggered
Doctors in Boston and in Mexico Have Life-Threatening Reactions to RNA COVID-19 Vaccines. Mexican Doctor Is Reported Paralyzed. https://needtoknow.news/2021/01/doct...d-19-vaccines/
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
THIS is well worth seeing. James is a researcher, who - if you listen carefully - is not anti-vaccine at ALL, and pro safe vaccines. He is and has been horrified by what is going on, tried to get information out to officials re: mRNA vaccines - in order to protect people and has been ignored. Talk can be a bit technical at first, but not all of it is and more is understood as talk goes on. There are also links to his research, papers, and more for greater understanding. Basically, people who get the vaccine - will be much much sicker when they are exposed to actual virus again. It hampers the immune system's function (he briefly explains how), may create auto-immune diseases so immune system attacks tissues and organs in body, etc.
@ 30 minutes: James describes his letter to Dr. Fauci - when Fauci announced he was coming out w/Zika vaccine... James listed the proteins in virus that matched proteins in us, Dr. Fauci never responded to James... but 3 days later said they'd need 8 more months before they could release Zika vaccine. Again, James had outlined all the human proteins that matched proteins in virus... that vaccine could make our immune system attack those areas of our nerves, our brain... and more.
Fauci was well aware of the reactions that people may get to PEG (polyethylene glycol) in mRNA vaccines because RFKJr informed him. Fauci decided to still use it because 'we have epi-pens'.... It was his choice and he should be held accountable for every anaphylactic reaction and death.
In a delayed response to these events, CDC - due to sheer numbers of anaphylaxis - is now saying if you've had this type of reaction before don't get the vaccine.
The affect on people - the irrational way we are handling the approach to this virus - goes way beyond the physical effects.... hundreds of thousands have lost jobs, businesses, etc. We need massive massive change and reform in our Public Health approaches.
James is asked how actual research should be carried out, rather than what is being done in race to market. He uses a hypothetical situation to illustrate....
He talks about regulators lobbied and funded by Big Pharma. James has a vision of how regulators could work
again for the public.... what that would look like (see his Plan B paper below)... It would decentralize Public Health, penalizes those in bed w/Pharma, and depoliticizes the process. We also need to kick Pharma out of politics.. Government has totally been co-opted.
In addition,Dr. James Lyons-Weiler Interview – The Politicization Of Medicine & The Dangerous Vaccine Agenda It’s Created "the idea of mass vaccination campaigns is not based on science, the vaccinologists have seized an unearned moral high ground. In reality, the depravity and disregard they exhibit for the sanctity of human life seems unmatched in modern times." Again, James says: "I am not anti-vaccine. I am pro-science. They have taken my baby - science, and injured my baby. I am raising two sons.. We're supposed to have an open free society (Karl Popper). To have CEOs of corporations say you cannot talk about this vaccine in the workplace - that is a closed society. It is a symptom of a process of thought called 'induction'. You are ruling by decree, making over-generalizations. It is devil-may-care, irresponsible, reckless. We need to reform everything we are doing around Medicine, Public Health so that Science can take hold again. Bioethics is dead in the U.S. The fact that some are calling for criminalization of speech around vaccines..... With Plan B... we don't have to fight them anymore; we are fighting FOR something of worth, rather than against something... I cannot imagine a police state future where we are tagged, all tracked at every moment -- at least not living under the U.S. Constitution. I would like everyone to look up the Federal Code of Regulations regarding Experimentation on Human Subjects. We are all entitled to say 'no, we don't want to be experimented on. We are all entitled to Free and Entire Informed Consent without coercion, specifically without coercion -- 100% without coercion. There are special protections for pregnant women, for children. That Federal Code of Regulations is there because of Nuremberg."
"the idea of mass vaccination campaigns is not based on science, the vaccinologists have seized an unearned moral high ground. In reality, the depravity and disregard they exhibit for the sanctity of human life seems unmatched in modern times." Again, James says: "I am not anti-vaccine. I am pro-science. They have taken my baby - science, and injured my baby. I am raising two sons.. We're supposed to have an open free society (Karl Popper). To have CEOs of corporations say you cannot talk about this vaccine in the workplace - that is a closed society. It is a symptom of a process of thought called 'induction'. You are ruling by decree, making over-generalizations. It is devil-may-care, irresponsible, reckless. We need to reform everything we are doing around Medicine, Public Health so that Science can take hold again. Bioethics is dead in the U.S. The fact that some are calling for criminalization of speech around vaccines..... With Plan B... we don't have to fight them anymore; we are fighting FOR something of worth, rather than against something... I cannot imagine a police state future where we are tagged, all tracked at every moment -- at least not living under the U.S. Constitution. I would like everyone to look up the Federal Code of Regulations regarding Experimentation on Human Subjects. We are all entitled to say 'no, we don't want to be experimented on. We are all entitled to Free and Entire Informed Consent without coercion, specifically without coercion -- 100% without coercion. There are special protections for pregnant women, for children. That Federal Code of Regulations is there because of Nuremberg."
https://www.thelastamericanvagabond....genda-created/
James’ Links:
https://www.thelastamericanvagabond....r11_2020.pages
https://twitter.com/lifebiomedguru
http://ipaknowledge.org/
Video Source Links:
https://ijvtpr.com/index.php/IJVTPR
https://jameslyonsweiler.com/2021/01...lth-now-has-a-plan-b/
https://jameslyonsweiler.com/wp-content/uploads/2021/01/Plan-B-IJVTPR-Volume-1-Issue-_2.pdf
https://pubmed.ncbi.nlm.nih.gov/32292901/
https://www.bitchute.com/video/rWjQALVvZEAH/
https://www.youtube.com/watch?v=FCyn-Ufjqoo
https://informedchoicewa.org/educati...nvaxxed-study/
https://www.youtube.com/watch?v=soIrKqnEykY
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
Ten Things You Need to Know about the Experimental COVID Vaccines
https://www.globalresearch.ca/10-thi...ccines/5734303
gives those 10 reasons and says:
 ...for those wishing to dig deeper, I suggest investigating things such as unsafe epitopes (parts of proteins capable of causing immune and auto-immune conditions), ADE (antibody-dependent amplification, long known from experiments with corona vaccines in cats. All cats that initially tolerated the vaccine well, died after catching the wild virus), nanoparticles (graphene and hydrogel) and more, all of which are likely linked to the COVID vaccines. These concoctions take the NWO scheme to a whole new level. The agenda has arrived at your doorstep and, indeed, at your bloodstream.
...for those wishing to dig deeper, I suggest investigating things such as unsafe epitopes (parts of proteins capable of causing immune and auto-immune conditions), ADE (antibody-dependent amplification, long known from experiments with corona vaccines in cats. All cats that initially tolerated the vaccine well, died after catching the wild virus), nanoparticles (graphene and hydrogel) and more, all of which are likely linked to the COVID vaccines. These concoctions take the NWO scheme to a whole new level. The agenda has arrived at your doorstep and, indeed, at your bloodstream.
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
Pfizer admitted the vaccine does not prevent Covid infection, and other vaccine problems; and how EMTs are being lied to and shamed to force vaccine uptake
MD gives excellent points re: these experimental agents.. then talks about issue of EMTs being lied to: People have asked why am I not blogging about the Covid vaccines. To be honest, I felt there was not enough information for me to be decisive, and I was waiting for more information to become available.
People have asked why am I not blogging about the Covid vaccines. To be honest, I felt there was not enough information for me to be decisive, and I was waiting for more information to become available.
However, someone called me this morning and told me about a lot of allergic reactions, including one anaphylactic reaction, at a local hospital after 30 doses were given. Staff were instructed to keep this quiet.
Today I watched a 9 minute Ben Swann video about the vaccines, in which he read the "Declination form" that must be signed by EMTs in Maine who refuse the vaccine. It contained false and misleading statements, and I realized I should no longer delay discussing what I know about the vaccines.
There are updates and comments worth reading at link as well This is a document designed to force EMTs to take the vaccine by using false information and veiled threats. For example, the document claims with certainty that one can asymptomatically spread Covid, even up to 10 days. That has not been shown to be true. Even Anthony Fauci was recorded as saying that asymptomatic spread has never driven an epidemic, although it might occur rarely. We still don't know with certainty how much asymptomatic spread contributes to cases, but probably very little.
This is a document designed to force EMTs to take the vaccine by using false information and veiled threats. For example, the document claims with certainty that one can asymptomatically spread Covid, even up to 10 days. That has not been shown to be true. Even Anthony Fauci was recorded as saying that asymptomatic spread has never driven an epidemic, although it might occur rarely. We still don't know with certainty how much asymptomatic spread contributes to cases, but probably very little.
CDC made a claim just this past week that asymptomatic spread could contribute to 59% of cases. CDC, however, made this claim based on its own researchers using modelling and estimates alone. CDC loves to publish its models of illness, cases and spread, instead of providing real data. Models can be easily manipulated to support whatever narrative is desired, as we have seen with the Neil Ferguson and University of Washington/BMGF models of the pandemic.
The declination document claims that the clinical trials were rigorous. I doubt few who read the trial documents would agree with that. The trials are still in progress. And FDA explicitly said these two vaccines have NOT BEEN APPROVED. They have instead been "authorized."
But the most pernicious thing about the EMT document was that it was intended to make the decliner feel awful for letting down the team and the community. In fact, based on the monkey data, the only data we have, you can still spread the virus even after being vaccinated. So the declination was built on a lie. And, lying document that it is, it is not signed. You don't know who wrote it. Why are EMTs being made to sign it, and initial every paragraph?
Here is just one of its passages:
"The consequences of my refusal to be vaccinated could have life-threatening consequences for my health and the health of everyone with whom I am in contact, including my co-workers, my family, and members of the community I serve."
When a product is good for you, there is no need to scare or threaten people into taking it. If you are being coerced to do something, that should be a strong clue to avoid it.
If you become injured by one of these experimental vaccines, the chance of receiving any financial benefit is tiny. The US government has waived the liability of everyone involved, from manufacturers to vaccinators.
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
The World Health Organisation (WHO) aware that 23 people in Norway died after being vaccinated with the Pfizer/BioNTech jab against coronavirus https://sputniknews.com/europe/20210...-follow-probe/
Belgian resident dies five days after Pfizer agent:
https://sputniknews.com/europe/20210...id-19-vaccine/
27-year-old mother suffers seizures, is hospitalized after taking COVID vaccine
UK government lies again: Starts funding COVID 'vaccine passports' just weeks after saying there were no plans for them
Lockdown Britain turning into Stasi-ruled East Germany? No, in many ways it's worse
After months of bashing Russia's vaccine, Western journalists in Moscow line up for Sputnik V
Russia's vaccine is CLEAN and "Despite Russia's vaccine having more thorough testing and not being based on the experimental, unproven and potentially dangerous mRNA gene-editing, it's worth remembering that for the vast majority of people no vaccine is needed..."
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
Calif. Calls For Pause On Batch Of Moderna's COVID Vaccine After Several Allergic Reactions
 The allergic reactions occurred at Petco Park’s mass drive-thru vaccination site
The allergic reactions occurred at Petco Park’s mass drive-thru vaccination site
No Evidence COVID-19 Vaccines Will Block Spread of Coronavirus
https://www.globalresearch.ca/no-evidence-covid-19-vaccines-block-spread-coronavirus/5734734
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
Informed Consent for COVID Vaccine
https://articles.mercola.com/sites/a...-vaccine.aspx?
 Significant concerns have been raised surrounding antibody‐dependent enhancement (ADE), and the possibility that COVID-19 vaccines could worsen COVID-19 disease via ADE
Significant concerns have been raised surrounding antibody‐dependent enhancement (ADE), and the possibility that COVID-19 vaccines could worsen COVID-19 disease via ADE
International Journal of Clinical Practice researchers called the risk of ADE in COVID-19 vaccines not only nontheoretical but also compelling
They noted that vaccine-elicited enhancement of disease has been previously found with SARS and Middle East respiratory syndrome-related (MERs) coronaviruses, as well as feline coronavirus, all of which are closely related to SARS-CoV-2, which causes COVID-19
Given the strong evidence of ADE risk from COVID-19 vaccines, the researchers believe that a separate informed consent form should be given out to those receiving the vaccine, warning them of the specific risk of worsened COVID-19 disease from vaccination
Despite researchers recommending back in October 2020 that ADE risk be “prominently and independently disclosed” to patients, no such warning exists
Informed consent to medical treatment is a right that ensures patients receive information about the recommended treatment so they can make a well-informed decision about their medical care.1 Medical practitioners are both ethically and legally obligated to ensure their patients have an opportunity for informed consent, which means disclosing both the risks and benefits of potential medical treatments.
In the case of the COVID-19 vaccine, it’s not possible to provide a full list of potential risks, considering the unprecedented speed with which they were developed and released to the public — the long-term effects are completely unknown.
China Health Experts Call for Suspension of COVID Vaccines as Norway Investigates 33 Deaths,
Germany Probes 10 Deaths
https://www.globalresearch.ca/china-...deaths/5734847
Israel confirms Pfizer jab 'less effective than thought', India warns vulnerable AGAINST getting AstraZeneca version
Woman who suffered convulsions after taking Pfizer Covid jab being screened for permanent neurological damage, son tells RT
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
British Medical Journal: Pfizer and Moderna’s “95% Effective” Vaccines—Let’s be Cautious and First See the Full Data https://www.globalresearch.ca/pfizer...data-2/5734637
 Only full transparency and rigorous scrutiny of the data will allow for informed decision making, argues Peter Doshi
Only full transparency and rigorous scrutiny of the data will allow for informed decision making, argues Peter Doshi
Pfizer says it recorded 170 covid-19 cases (in 44,000 volunteers), with a remarkable split: 162 in the placebo group versus 8 in the vaccine group. Meanwhile Moderna says 95 of 30,000 volunteers in its ongoing trial got covid-19: 90 on placebo versus 5 receiving the vaccine, leading both companies to claim around 95% efficacy.
Let’s put this in perspective.
First, a relative risk reduction is being reported, not absolute risk reduction, which appears to be less than 1%.
Second, these results refer to the trials’ primary endpoint of covid-19 of essentially any severity, and importantly not the vaccine’s ability to save lives, nor the ability to prevent infection, nor the efficacy in important subgroups (e.g. frail elderly). Those still remain unknown.
Third, these results reflect a time point relatively soon after vaccination, and we know nothing about vaccine performance at 3, 6, or 12 months, so cannot compare these efficacy numbers against other vaccines like influenza vaccines (which are judged over a season).
Fourth, children, adolescents, and immunocompromised individuals were largely excluded from the trials, so we still lack any data on these important populations."
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
Operation Coronavirus is Working Hand-in-Hand with the Nanotech Agenda
the above article looks at nanotechnology prior to and related to mRNA agents. There are links to author's other articles looking at this trend and its history over many years. There is also a link of a 2017 study done in Italy - looking at contents of vaccines - showing nanotech ingredients:
There are links to many other nanotechnology-related articles. The study (which analyzes 44 types of 15 traditional vaccines) states that these contaminants are “non biodegradable and non biocompatible”, that their inclusion is “not declared” and that their presence is “inexplicable.”
The study (which analyzes 44 types of 15 traditional vaccines) states that these contaminants are “non biodegradable and non biocompatible”, that their inclusion is “not declared” and that their presence is “inexplicable.”
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
 Merck Scraps Covid 'vaccines'; Says It's More Effective To Get Virus and Recover: The company announced that the shots V590 and V591 were ‘well tolerated’ by test patients, however they generated an ‘inferior’ immune system response in comparison with natural infection. https://www.merck.com/news/merck-dis...ic-candidates/
Merck Scraps Covid 'vaccines'; Says It's More Effective To Get Virus and Recover: The company announced that the shots V590 and V591 were ‘well tolerated’ by test patients, however they generated an ‘inferior’ immune system response in comparison with natural infection. https://www.merck.com/news/merck-dis...ic-candidates/
*******
Peer-reviewed Medical Journal Data: Can Flu Vaccine Increase COVID Risk?
https://articles.mercola.com/sites/a...ovid-risk.aspx
Discrepancies in Moderna’s FDA Report Demand Answers previous flu vaccination seems to increase patients' risk of contracting more severe pandemic illness. This occurred during the 2008 to 2009 flu season
previous flu vaccination seems to increase patients' risk of contracting more severe pandemic illness. This occurred during the 2008 to 2009 flu season
...A January 2020 study published in the journal Vaccine also found people were more likely to get some form of coronavirus infection if they had been vaccinated against influenza during the 2017 to 2018 flu season.
October 2020, another positive association was found between COVID-19 deaths and flu vaccination rates in the elderly,
Moderna’s reported death rate for its COVID vaccine, based on clinical trials, is 5.41 times greater than Pfizer’s. Yet neither are representative of national death rates — that’s a red flag.
https://www.globalresearch.ca/discre...nswers/5735484
Helsinki Committee: Israeli government's and Pfizer's illegal experiment on humans
https://jewishbusinessnews.com/2021/...humans-report/
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
Invaluable information in article:
The Inanity of RNA Vaccines For COVID-19
 The present article is a follow-up of Compelling Evidence That SARS-CoV-2 Was Man-Made published in June 2020, you are encouraged to read it first.
The present article is a follow-up of Compelling Evidence That SARS-CoV-2 Was Man-Made published in June 2020, you are encouraged to read it first.
The NYT’s Has Not Heard of China’s Vaccine (Or Russia’s or India’s)
RNA ‘Vaccines’ Are GMO Implants, Not Vaccines
https://needtoknow.news/2021/01/rna-...-not-vaccines/
-
-
 M/M
M/M
-
Real Name: (not displayed to guest users)
-
Join Date: May 9, 2017
- Expressed Gratitude: 1,085
- Received Gratitude 589 times for 315 posts
-
Last Online 02-08-2021
- View Profile
-
 Ignore
Ignore
-
 View Posts: (431)
View Posts: (431)
Re: More about vaccines
Germany says AstraZeneca COVID-19 vaccine isn’t for people 65 and older (18-64 okay)
https://nypost.com/2021/01/28/german...65-and-older/?
USG buys too much vaccine. Governments Sign Secret Vaccine Deals: Here’s What They Hide/ NYT
Israel and Pfizer just discovered facial nerve (Bell's) palsy is a side effect of Pfizer's shot/ We knew it December 17 The US government is buying 600 million Covid vaccine doses from Moderna (alone) and has contracts to purchase perhaps 1.5 billion more doses. But it also has signed contracts restricting the overseas sale of some of this. So if it can't be sold, why is the government committed to buying much more vaccine than Americans need? Or is the plan in place to give everyone frequent boosters? The contracts are uber secret. Multibillion-dollar contracts give drug makers liability shields, patent ownership and leeway on delivery dates and pricing — and promises that much of it will not be made public.
The US government is buying 600 million Covid vaccine doses from Moderna (alone) and has contracts to purchase perhaps 1.5 billion more doses. But it also has signed contracts restricting the overseas sale of some of this. So if it can't be sold, why is the government committed to buying much more vaccine than Americans need? Or is the plan in place to give everyone frequent boosters? The contracts are uber secret. Multibillion-dollar contracts give drug makers liability shields, patent ownership and leeway on delivery dates and pricing — and promises that much of it will not be made public.
Did CDC Deliberately Mislead the Public on Anaphylaxis reactions to Moderna's Covid vaccine? https://childrenshealthdefense.org/d...derna-vaccine/
X-ray tech dies after 2nd Covid shot...
https://khn.org/morning-breakout/cal...eived-vaccine/
-
-
Mayacaman

-
Real Name: (not displayed to guest users)
-
Join Date: Jan 17, 2019
- Expressed Gratitude: 670
- Received Gratitude 901 times for 556 posts
-
Last Online 03-26-2023
- View Profile
-
 Ignore
Ignore
-
 View Posts: (1,222)
View Posts: (1,222)
Re: More about vaccines
* 7 die at Spanish care home after getting Pfizer Covid-19 jab as ^All 78 residents at a nursing home in central Spain have tested positive for Covid-19 after being given their first dose of the Pfizer-BioNTech vaccine, and at least seven people have died, staff confirmed on Monday.
ALL residents test positive for virus, second doses still to come
by RT.com
Excerpts:
Most of those who succumbed to the virus had existing conditions, according to Spanish news agency EFE, while four residents are currently hospitalized, and 12 staff have also been infected.
The huge outbreak is at the Lagartera Residence for the Elderly in the Toledo area, southwest of the capital Madrid.
The home’s 33 staff must now present a negative PCR test before they start work, and a spokesperson said that health measures to contain the spread of the virus are in place “at all times.”
“On January 13, all residents, including nursing home staff, were vaccinated with the first dose of the Pfizer vaccine, and after six days the first symptoms began to appear in ten of the residents,” they said in a statement.Some members of staff began to go off sick with the virus five days after being inoculated.
On January 21, management approved the decision to test all residents of the home and quarantine them to their rooms, with families informed of the move.
The testing results, on January 25, showed that all the residents had caught the virus apart from one, who then also tested positive at a later date.
In December, Spain’s Supreme Court ordered an investigation into deaths at nursing homes, which were a disturbing feature of the early pandemic, making up 69 percent of all Covid-19 fatalities between April 6 and June 20.
The Lagartera Residence for the Elderly insisted the current outbreak was its first of the pandemic, having remained virus-free during the first two waves of infections.
The next doses of the vaccine are to be administered at the home on February 3, and the next round of PCR tests will be carried out on February 5
Read the full article at RT.com.
-
-
Mayacaman

-
Real Name: (not displayed to guest users)
-
Join Date: Jan 17, 2019
- Expressed Gratitude: 670
- Received Gratitude 901 times for 556 posts
-
Last Online 03-26-2023
- View Profile
-
 Ignore
Ignore
-
 View Posts: (1,222)
View Posts: (1,222)
Re: More about vaccines
 Are More People Being Harmed by the Pfizer Experimental “Vaccine” than from COVID? Israeli Population Now the World’s Lab Rats Sold Out to Pfizer
Are More People Being Harmed by the Pfizer Experimental “Vaccine” than from COVID? Israeli Population Now the World’s Lab Rats Sold Out to Pfizer
NOTE :
Normally, I would have posted this in the Uptown "Coronavirus" Ward, but as of yesterday & after my latest posts, I have been notified by Mr. Chertov that I may no longer post up there with the proper White folks. So I am posting the following item down here in the Ninth Ward. It is after all, just hearsay and shall probably - no doubt - be written off as merely one more "conspiracy theory." But the fact that the Jews of Israel have been sold down the river by Net-one-yahoo, and are being used as lab-rats in an experiment by a German Pharmaceutical-Industrial Corporation, ought to Sound a few alarms - in a few minds, at least...
Government Consigned Israeli PopulationBBC: “Israel bought large stocks of the [Covid-19] jab in exchange for acting as the world’s guinea pig.”
to be Human Subjects in a Massive Experiment
by Vera Sharav
Alliance for Human Research Protection
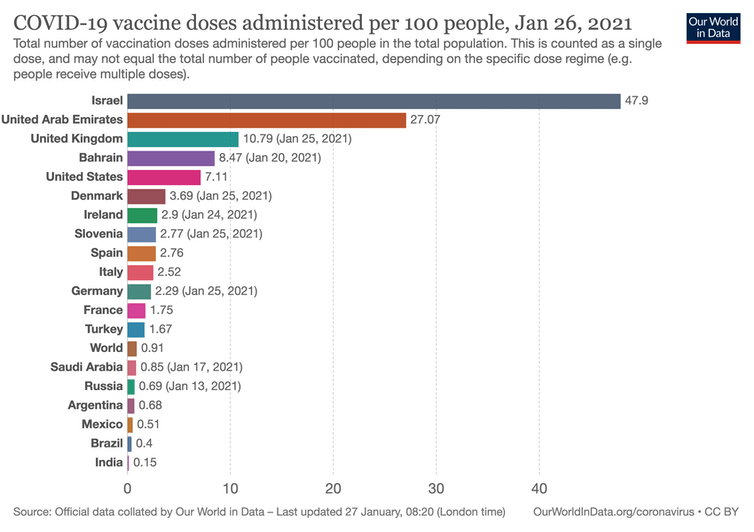
On November 18, 2020, Israel’s senior health officials were caught unprepared when Pfizer announced that its vaccine was “90% effective” (revised to 95%) against Covid-19.
They had ordered millions of vaccine doses from Moderna and AstraZeneca but none for the Pfizer-BioNTech vaccine.
How then, did Israel procure an estimated four to five million doses of the Pfizer vaccine in December 2020 – enough to vaccinate at least two million people?
Prime Minister Binyamin Netanyahu sought to show that he singlehandedly saved the day; as did Donald Trump when he launched Operation Warp Speed, to accelerate the production and distribution of Covid-19 vaccines. Both heads of state entered into secret contracts with vaccine manufacturers. Both failed to disclose to their constituents that the COVID-19 (mRNA) vaccines are experimental.
- The COVID-19 vaccine phase 3 clinical trials have not yet been completed. This is why both the Pfizer and Moderna mRNA vaccines have NOT been licensed by the FDA. Both vaccines received “Emergency Use Authorization” (EUA): “a mechanism to facilitate the availability and use of medical countermeasures…Under an EUA, FDA may allow the use of unapproved medical products.”[i]
- The safety of these experimental vaccines has not been established during the frenzied pace of production and distribution. The sheer speed at which the vaccines were developed and tested precluded obtaining sufficient information about adverse side effects; in particular, serious, long term adverse effects.
- Furthermore, mRNA technology used in these vaccines is also experimental.[ii] No other vaccines using this technology have been approved. The long-term risks are unknown.
- As of January 27, 2021, Johns Hopkins’ tracking system states that the infection fatality rate in the U.S. is 1.7%; in Israel it is 0.7%.[iii]
According to the US Centers for Disease Control (CDC), as of Dec. 18, 2020 (just when mass vaccinations began), the adverse event rate following Covid-19 vaccination was 2.79%.[iv]
This would indicate that the short-term risk of harm from the vaccine is far greater than the risk of dying from COVID-19.
- On December 10th, at FDA’s advisory committee meeting to evaluate Pfizer’s vaccine for use under EUA, Dr. Kathrin Jansen, Pfizer’s lead representative, acknowledged that despite being vaccinated with Pfizer’s vaccine, monkeys became infected when they were exposed to the virus.[v] Pfizer did not test whether vaccinated people could also become infected when exposed to the virus. It is, therefore, entirely possible that the Covid-19 vaccine is no defense against infection.
- On January 26 2021, the World Health Organization posted the latest news about the Moderna Covid-19 vaccine: What You Need to Know, in which the WHO confirmed lack of efficacy evidence: “We do not know whether the vaccine will prevent infection and protect against onward transmission. Immunity persists for several months, but the full duration is not yet known. These important questions are being studied.”[vi]
- The acknowledged lack of evidence for the protective value of both of the mRNA vaccines refutes the widely publicized “90% -95% effectiveness” claims. The absence of evidence for the protective value of these vaccines eliminates the justification for exposure to the risks; and undermines the claimed need for these vaccines.
The high degree of panic generated by continuous, fear-generating claims about the virus, led public health officials to discard the precautionary principle in medicine – “First, do no harm”.
Despite serious uncertainty, officials proceeded full speed ahead, with mass vaccination.
Israel’s fast-track vaccination programme is providing data for the world on efficacy. Abir Sultan/EPA – Source.
It is astonishing that the government of Israel entrusted the health of the people to Pfizer; by entering into a secret contract that enrolled the Israeli population to become research subjects, without their knowledge or consent.
Under the contract, Real World Epidemiological EvidenceCollaboration Agreement,[vii] the government signed a commitment to vaccinate the entire seven million adult population and to provide weekly data on its citizens during a 24-month surveillance follow-up study.
The government disregarded potentially serious medical risks from the experimental vaccine and risks to privacy.
Israel is considered an ideal place for a vast epidemiological study, encompassing 9.3 million people, because of its universal, state-sponsored healthcare system in which insurers maintain 40 years of digitized medical records, including vaccination records for each Israeli citizen.
This centralized system helped Israel administer more than 2 million doses of the vaccine in under a month. In exchange, Israel received priority delivery of millions of doses of the vaccines.
Netanyahu stated that the reason Israel received so many vaccine doses so quickly is that:
“Israel has committed to send Pfizer data and details especially gathered for them, including the consequences of the inoculations, side effects, efficacy, amount of time it takes to develop antibodies according to different types of population, age, gender, preexisting conditions etc. The agreement extensively details the various parameters that will be sent to Pfizer.”[viii]Former Prime Minister Ehud Barak pointed out that Pfizer is using the Israeli population as a “perfect testing ground” for its COVID-19 vaccine.
“This data is a treasure trove for Pfizer. It’s a huge asset to Pfizer, because it lets them show that when someone dies after being vaccinated – and people my age tend to die more often – he didn’t die because of the vaccine, but as a result of some background illness.”[ix]
- Israeli citizens became the unwitting human subjects of a massive, unethical, unapproved, non-consensual human experiment. The public was not informed that (a) the vaccine is experimental; (b) the population was being used as human subjects for a two-year epidemiological study; the data is meant to be shared with foreign countries and journals; the secrecy of the contract has led to strong suspicions that (c) their personal medical record – “a data treasure-trove” – would be shared with Pfizer.
“In effect, Bibi [Netanyahu] has signed up his people, all seven million citizens aged 12 years and over, without our informed consent, to become the first country in its entirety to do human testing on a technology which has been, for many decades, attempted and failed in the laboratory…Our citizens must first and foremost define the discussion in order to accurately weigh the choices…[We] have been given little information at all and that includes complete opacity of data on the unfolding outcomes of adverse reactions currently taking place.”[x] [Ilana Rachel Daniel, Arutz Sheva, Israel National News]Pfizer seeks to obtain basic safety and efficacy information that it lacks, in order for its vaccine to be eligible for an FDA license. No vaccine has ever before been administered to millions of people without having met safety and efficacy requirements.
The following information must normally be obtained during controlled clinical trials prior to public distribution.
First, the company needs to demonstrate that the vaccine is effective in preventing infection when one is exposed to the virus.
Second, serious adverse effects need to be identified and their frequency and duration determined.
Third, the risk for specific populations, including children, pregnant women and the elderly need to be identified and weighed against the benefits.
Fourth, causes of deaths during the trial need to be documented.
On January 19, 2020, the Israeli newspaper Haaretz reported that over 12,400 Israelis who had been vaccinated tested positive for Covid – that’s 56.6% of the 189,000 vaccinated people.
Dr. Nachman Ash, Israel’s national pandemic coordinator, warned on Army Radio that:
“Many people have been infected between the first and second injections of the vaccine. The protective effect appears “lower than we thought [and] lower than [the data] presented by Pfizer.”He warned that restrictions will continue longer than expected. “With COVID-19 mutations, third lockdown may not be Israel’s last.”
Should pregnant women be exposed to a controversial, experimental vaccine?
The safety of the vaccine has not been adequately ascertained under Operation Warp Speed. It has never been tested in pregnant women.
On January 8th the W.H.O. advised against vaccinating pregnant and breastfeeding women” “due to insufficient data, WHO does not recommend the vaccination of pregnant women at this time.”[xi]
The C.D.C. issued conflicting advice:[xii] The WHO came under pressure two days after the media reported the conflicting advice.[xiii] Citing no new data, the WHO rescinded its cautionary guideline stating: “based on the data at hand, we don’t have any….reason to believe there are specific risks…”
However, the WHO continues to warn that data on Covid-19 vaccines and pregnancies is lacking, making safety assessments difficult.[xiv]
Those who regard vaccines as a Holy Grail – throw caution to the wind. Have they forgotten the disastrous tragedies that followed when pregnant women were prescribed Thalidomide[xv] and Diethylistilbestrol (DES)?
Vaccine zealots disregard medicine’s precautionary “do no harm” principle before applying any invasive medical intervention. They blame the lack of data on the precaution against exposing expectant mothers to clinical trials.
Pfizer’s experiment in Israel will fill the gap. Dr. Ash has not only recommended the vaccine for pregnant women, he added pregnant Israeli women to the priority vaccination list.[xvi]
“Biological Experiment”
- Pregnant Israeli women have not been informed that the vaccine is experimental. Neither are they aware that they are being consigned – like rabbits – in a massive medical experiment without their informed consent.
Pfizer, the company to whom the Prime Minister and officials of the Department of Health entrusted to test its experimental vaccine on the entire population of Israel, is an unscrupulous business corporation with an extensive Rap Sheet compiled by Corporate Research Project.[xvii]
Pfizer’s track record is filled with cases in which it was accused of misleading regulators and the public about the safety of its products.
One of the most notorious cases of criminal human rights violations involved Pfizer’s unapproved clinical trial conducted in Nigeria.
Pfizer enrolled 200 children to test its new, experimental antibiotic drug Trovan, deceiving parents by falsely claiming that it was an approved therapy for meningitis.
In fact, Pfizer sought to obtain data so that their drug could get an FDA approval; a process that should have taken at least a whole year (or longer) was rushed through in six weeks — much like the testing process for its Covid-19 vaccine was rushed.
The Trovan trial resulted in the death of eleven children, and twelve others were left permanently disabled. Two years later the FDA warned that the drug could cause liver damage and death.
The mass vaccination experiment is being conducted in violation of Israel’s legal ethics requirements; namely, review and approval by the Institutional Review Board / Ethics (Helsinki) Committee.[xviii]
The Helsinki Committee approval is required for any research study involving human subjects and is also required for any deal by the Israeli government that provides citizens’ data to other entities, especially if they are foreign.
A senior official of the Helsinki Committee confirmed the “clear, unequivocal and unambiguous evidence that the contract with Pfizer is a clinical study”:
“Reading the contract signed between the Israeli government and Pfizer, it is clear, unequivocal and unambiguous that this is a clinical study for all intents and purposes, and thus, it needed to be approved by the Helsinki Committee. And that’s what will be written in the Committee’s opinion:Senior Israel Democracy Institute attorney Dr. Tehila Schwartz-Altshuler described: the experiment in Calcalist: “This is the most extensive study of human beings in the 21st century.
‘There’s nothing wrong with clinical trials, on the contrary, but clinical trials (human trials) must get committee approval, and of course, from the people on whom the trial is being conducted, while giving the right to refuse to be part of the trial. These are very basic matters.‘”[xix]
- The experiment violates the Nuremberg Code;[xx] the most important document in the history of medical research ethics standards. The foremost ethical principle of the Nuremberg Code is as relevant today as it was in 1947: “The voluntary, informed consent of the human subject is absolutely essential.”
- Informed consent is “absolutely essential” because it affirms the human right of the individual to accept or reject. Informed consent stands as a moral/legal barrier to ensure that governments “Never Again” pervert medicine.
- “The right to refuse” a medical intervention is reaffirmed under the Universal Declaration on Bioethics and Human Rights(2005) which states: “Any preventive, diagnostic and therapeutic medical intervention is only to be carried out with the prior, free and informed consent of the person concerned, based on adequate information.”[xxi]
- Medicine is not like a commodity; medical decisions are personal, requiring the weighing of benefits against risks. Medicine often involves life and death decisions. Without informed consent of the individual, medicine can be, and has been, weaponized. Under Operation Warp Speed, the dangerously abbreviated clinical trials were far too short to document the scope or severity of vaccine adverse effects.
Both vaccines are made from messenger RNA and lipid nanoparticles containing polyethylene glycol (PEG). Scientists believe PEG poses a risk of anaphylaxis[xxiii].
The risks include: severe allergic reactions, such as, anaphylaxis; systemic inflammatory response syndrome;[xxiv]autoimmune disease;[xxv] and antibody-dependent enhancement.
The latter is the focus of a recent peer reviewed report in the International Journal of Clinical Practice, by scientists from New York University and Tulane who warn that:
THE RISK OF ADE IN COVID‐19 VACCINES IS NON‐THEORETICAL AND COMPELLING
COVID‐19 vaccines designed to elicit neutralising antibodies may sensitise vaccine recipients to more severe disease than if they were not vaccinated.
The prior evidence that vaccine‐elicited, antibody‐dependent enhancement (ADE) of disease is likely to occur to some degree with COVID‐19 vaccines is vertically consistent from controlled SARS studies in primates to clinical observations in SARS and COVID‐19.
Thus, a finite, non‐theoretical risk is evident in the medical literature that vaccine candidates composed of the SARS‐CoV‐2 viral spike and eliciting anti‐SARS‐CoV‐2 antibodies, be they neutralising or not, place vaccinees at higher risk for more severe COVID‐19 disease when they encounter circulating viruses.
The other major issue of concern is the violation of the right to PRIVACY.
- Israel’s Health Ministry claims that it had obtained all required approvals; clearly this is not true.
The transfer of extensive, sensitive medical data to a multi-national foreign corporation exposes every Israeli citizen to extraordinary risks of harm. The most important commodity today is data. The transfer of its citizens’ medical data exposes the State of Israel to security risks.
Pfizer is one of the most unscrupulous business corporations whose court-adjudicated corrupt and illegal business practices includes numerous product safety violations, bribery in eight countries, and other crimes too numerous to be reviewed here.
A 27-year record of pharmaceutical company criminal and civil violations that led to settlements with U.S. state and federal government, was compiled by Public Citizen.[xxvi] Pfizer has the dubious distinction of setting two records; Pfizer’s corrupt business practices resulted in the highest criminal fine in US history for $2.3 billion, and the largest civil fraud settlement of $1 billion.
This is the company to whom Prime Minister Netanyahu and Israel’s Department of Health entrusted with the data of its citizens’ personal medical records. That data contains a treasure trove of information that Pfizer might even sell to the highest bidder.

Attorney Tehila Schwartz-Altshuler
Attorney Tehila Schwartz-Altshuler of Israel Democracy Institute expressed serious concerns about the secret contractual provisions; which she strongly suspects, provide Pfizer with anonymized personal data of citizens’ medical records from which names, addresses and ID numbers are removed. However, she noted that:
“One of the crucial problems with the agreement is that although it acknowledges the need to preserve anonymity and privacy of Israelis, it does not outline steps to protect this principle…and there are loopholes, such as secondary uses of data. ”Jonathan Klinger, a cyberlaw attorney and legal adviser for the Israeli Digital Rights Movement, a nonprofit group, agrees: “we don’t really know what’s being shared. Even if aggregated or anonymous data is transferred, it could be re-identified. This is still a concern.”
“The problem is that technology today is so advanced that research has shown that even data that has been rendered anonymous can be “de-anonymized. [It is a] huge risk.”[xxvii]
“Bibi feels that my health data belongs to him. If you give someone else or give away my medical records — which are the most sensitive kind of data that someone can know about me — you need my permission. Why didn’t you ask for my permission? Treating this personal data as if it belongs to the government is “not ethically, not legally and not morally right.”” [TSA]The Helsinki Ethics Committee has yet to issue its ruling about the experiment and the transmission of personal health data to Pfizer without the specific agreement of every individual person who is a de facto subject.
Read the full article at AHRP.org.
Comment on this article at HealthImpactNews.com
See Also:
12,400 People in Israel Tested Positive for Coronavirus AFTER Being Injected with the Experimental Pfizer COVID Shot
4 People Died and 240 Got COVID19 in Israel After Being Injected with Pfizer Experimental mRNA Vaccine
-
Gratitude expressed by 2 members:
Gratitude expressed by:
Gratitude expressed by:
Gratitude expressed by 3 members:
Gratitude expressed by:
Gratitude expressed by:
Gratitude expressed by:
Gratitude expressed by:
Gratitude expressed by:
Gratitude expressed by:
Gratitude expressed by:
Gratitude expressed by:
Gratitude expressed by:
- Site Areas
- Settings
- Private Messages
- Subscriptions
- Who's Online
- Search Categories
- Categories Home
- Categories
- Sonoma County Bulletin Board
- General Community
- Coronavirus
- Coronavirus Conspiracy Theories
- Events, Classes and Meetings
- Business Directory
- Sales & Timely Offers
- Services/Referrals Wanted
- Health & Wellness
- For Sale/Free/Wanted
- Employment Offered & Wanted
- Housing/Offices
- WaccoElders
- Housesitting/Petsitting
- Pets and other Critters
- Marin County Bulletin Board
- Discussion Board
- About WaccoBB
Similar Threads
-
Vaccines Cause Adulthood
By Finell in forum Coronavirus Conspiracy TheoriesReplies: 0Last Post: 08-11-2020, 03:28 AM -
Vaccines are a medical need
By Valley Oak in forum CoronavirusReplies: 2Last Post: 05-03-2020, 10:28 AM -
Issues around vaccines
By M/M in forum WaccoReaderReplies: 4Last Post: 04-10-2020, 09:26 AM -
Vaccines/Autism?
By Sylph in forum WaccoReaderReplies: 2Last Post: 02-03-2009, 10:27 AM -
Vaccines: Good or Bad?
By HolisticKids in forum WaccoTalkReplies: 37Last Post: 10-29-2006, 11:09 PM
Bookmarks
-
 Facebook
Facebook
-
 Twitter
Twitter
-
 StumbleUpon
StumbleUpon